Differences Among Electroplating, Electrophoresis and Painting in badges custom Surface Treatment Processes
Process Differences
1. Electroplating
It refers to the deposition of polymer resin on metal surfaces. It is a process of plating a thin layer of other metal or alloy onto the surface of certain metals using the principle of electrolysis.
2. Electrophoresis
Electrophoresis involves the movement of charged paint ions to the cathode under the voltage applied between the anode and cathode, reacting with the alkaline substances generated on the cathode surface to form insoluble deposits on the workpiece surface. Under the action of an external DC power supply, colloidal particles move directionally toward the cathode or anode in the dispersion medium, which is called electrophoresis.
3. Painting
Baking varnish refers to spraying multiple layers of paint onto the polished product, which is then shaped by high-temperature baking.
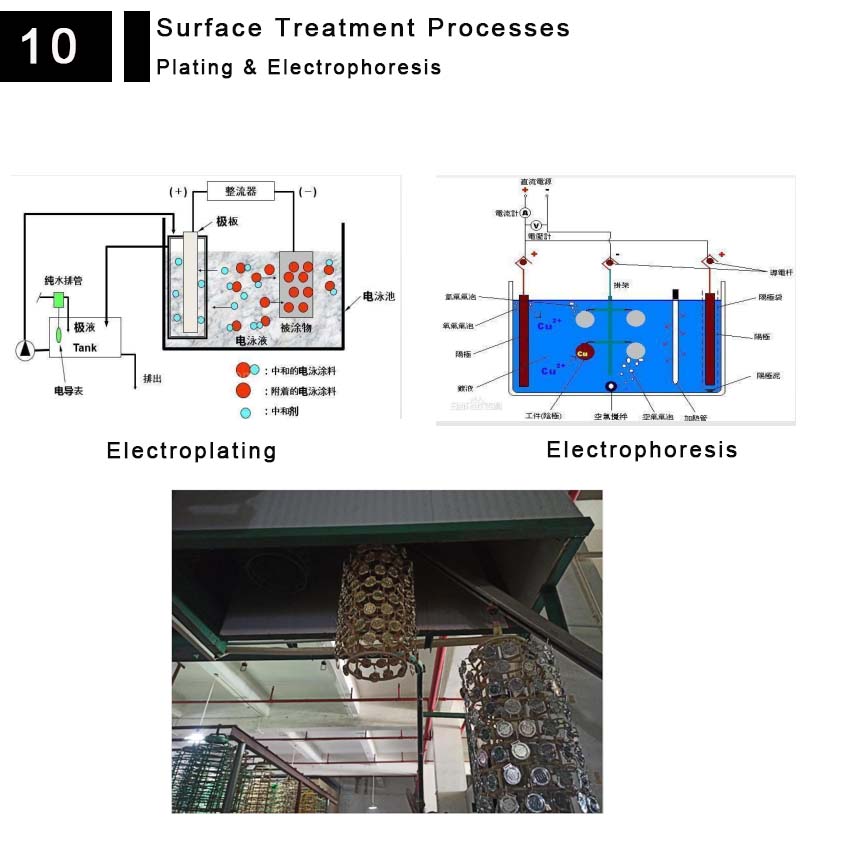
surface treatment plating electrophoresis
Cost Differences
Electroplating > Painting > Electrophoresis
Functional Differences
1. Electroplating
It uses electrolysis to coat the surface of metal or other materials with a metal film, serving purposes such as preventing metal oxidation (e.g., rust), improving wear resistance, electrical conductivity, and enhancing aesthetics.
2. Electrophoresis
It has advantages in film coverage, as the electrophoretic coating can completely cover hidden areas.
Surface Material Composition
1. Electroplating
The treated surface is a metal layer, such as copper plating, nickel plating, gold plating, silver plating, etc.
2. Electrophoresis
Pigments and resin particles in the electrophoretic solution migrate directionally and deposit on the surface of one of the electrodes.
3. Painting
The surface is sprayed with epoxy resin paint.